29 . 03 . 2021
The Gut-Brain Axis: a two-way street
The bi-directional communication between the central nervous system and the gut is increasingly accepted and supported from a scientific point of view. In this article, we will explore the mechanisms behind this relationship.
As we learn more about our own biology, and the way that different ecosystems work in symbiosis in the human body, it is possible to establish new connections and explore potential therapeutic avenues. One of the areas of major focus has been the intestinal microbiota, which is the main reservoir of microorganisms in the human body.
How can the microbiota influence the brain?
We can say that what goes on in the gut does not stay there. The microorganisms that inhabit the gut, participate actively in the digestion process, helping us to produce and absorb certain nutrients a. The microbiota can influence the brain through the following ways:
-
production of nutrients and vitamins – for example B vitamins – which have a direct effect on energy production and neuronal function (1).
-
production of neurotransmitters, which may have local or remote activity. For example, the neurotransmitter GABA is produced by several bacteria of the commensal flora, and has a direct effect on anxiety states (2). Serotonin levels are also modulated by the intestinal flora.
-
preservation of the intestinal epithelial barrier function. The intestinal mucosa should function as a barrier with selective permeability, keeping substances with potential toxicity out of circulation. A healthy and balanced intestinal flora helps maintaining the barrier function. Elevated levels of lipopolysaccharides (LPS) have been associated with a high risk of depression, for example (3). Lipopolysaccharides, also called endotoxins, are fragments of the membrane of certain bacteria, which have an inflammatory effect, and can be absorbed if there is an increase in intestinal permeability.
-
short-chain fatty acids synthesis – these are products of the metabolism of certain bacteria of the commensal flora, which have an anti-inflammatory effect at the local and systemic level. Recent studies have suggested an inverse relationship between the levels of butyrate (one of the short-chain fatty acids) and the presence of amyloid plaque in the brain, which is characteristic of Alzheimer’s disease (4).
And how does the brain influence the gut?
Probably, we have all felt the impact of our emotional state on digestion, in different ways. There is a neural network that directly coordinates the motility and secretory function of the gut – the enteric nervous system. In turn, this system receives inputs from the autonomic nervous system (sympathetic and parasympathetic) and from the hypothalamic-pituitary-adrenal axis (HPA), which controls the neurohormonal response to stress. Basically, there is a dense neural matrix that establishes the connection between what is processed in the brain and what happens in the gut.
This modulation focuses on the following functions:
-
changes in intestinal motility, due to imbalances between the two branches of the autonomic nervous system (sympathetic and parasympathetic).
-
secretion of digestive enzymes and mucus. The mucus produced by the epithelial cells has a protective effect and also promotes balanced community of symbiotic bacteria. This secretion is directly affected by the autonomic nervous system (5).
-
increased intestinal permeability induced by stress, through the stimulation of the HPA axis, with the production of CRF (Corticotropin-releasing factor) (5). As mentioned above, this increase in intestinal permeability can lead to endotoxin absorption, which carries systemic repercussions.
-
promotion of visceral hypersensitivity. Certain emotional states and chronic stress can lead to a decrease of the intestinal pain threshold. This is an important mechanism in functional gastrointestinal diseases, such as the common Irritable Bowel Syndrome (6).
All of these mechanisms are not limited and influence the microbiota in different ways, generating a cycle. For instance, in cases of hyperactivation of the HPA axis and the sympathetic branch of the ANS (autonomic nervous system), there is secretion of noradrenaline and other neurotransmitters in the gut, which in turn promotes the growth of certain bacteria.
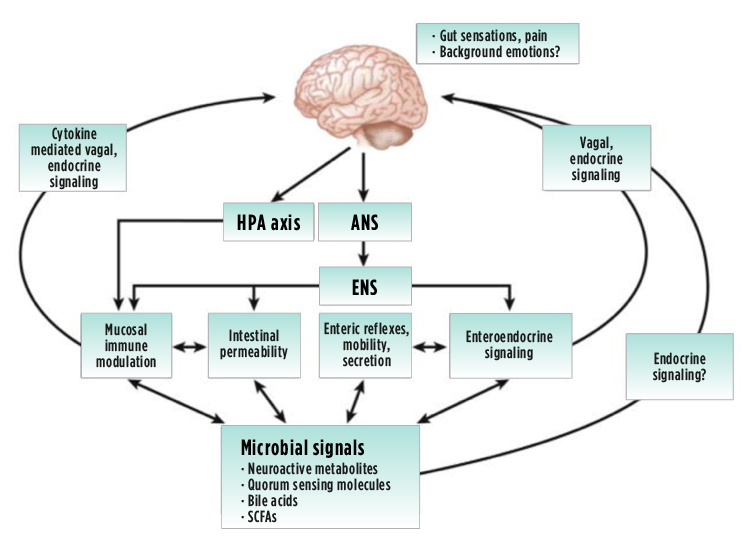
Fig. 1 – Signalling pathways between the brain and the gut (6). HPA axis – Hypothalamic-Pituitary-Adrenal axis; ANS – Autonomic Nervous System; ENS – Enteric Nervous System
How does this relationship affect treatment?
Given this complex and bidirectional relationship, we can divide the available therapies according to their main target:
1. Central nervous system
This category includes methods to mitigate any imbalances that may be contributing to intestinal dysregulation. These methods may be of pharmacological or other origin. One modality of particular interest is Gut-Directed-Hypnotherapy. In this type of intervention, the patient is guided to process sensory stimuli in a different way, in order to modulate the neurological response at the intestinal level. In a 2016 study, this approach was found to be as effective as the low-FODMAP diet in controlling symptoms of Irritable Bowel Syndrome (7). Gut-Directed-Hypnotherapy does not seem to significantly affect the intestinal microbiota, which suggests a different mechanism of action (8).
2. Gut
This includes substances commonly used in the treatment of functional gastrointestinal disorders, which act mainly on motility and secretory activity. There are pharmacological and natural alternatives, such as peppermint extract, which has an anti-spasmodic activity and is mentioned in the latest guidelines of the American College of Gastroenterology, for the treatment of Irritable Bowel Syndrome (9).
3. Microbiota
It is possible to achieve good clinical results in functional diseases of the gastrointestinal tract through modulation of the microbiota. There are several ways to “adjust” the microbiota:
- diet – fiber and polyphenol rich foods help promoting a healthy microbiota
- use of prebiotics – substances that feed and promote the growth of certain bacterial strains.
- probiotics – administration of microorganisms that have a specific and studied action. Recently, the term “psychobiotic” was introduced, which refers to specific probiotic strains capable of altering the production of neurotransmitters in the intestine (10).
- antibiotics – direct intervention with substances with antibiotic activity may be necessary. For example, the use of rifaximin in the treatment of Small Intestine Bacterial Overgrowth (SIBO).
In this brief article, we can attest to how complex the interrelationship between the brain and the gut is, and the way they affect each other. These associations make the understanding less linear, but, at the same time, open new possibilities for complementary clinical interventions.
References:
1. Yoshii K, Hosomi K, Sawane K, Kunisawa J. Metabolism of Dietary and Microbial Vitamin B Family in the Regulation of Host Immunity. Front Nutr. 2019 Apr 17;6:48.
2. Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018;1693(Pt B):128-133.
3. Liang S, Wu X, Jin F. Gut-Brain Psychology: Rethinking Psychology From the Microbiota-Gut-Brain Axis. Front Integr Neurosci.
4. Marizzoni M, Cattaneo A, Mirabelli P, Festari C, Lopizzo N, Nicolosi V, Mombelli E, Mazzelli M, Luongo D, Naviglio D, Coppola L, Salvatore M, Frisoni GB. Short-Chain Fatty Acids and Lipopolysaccharide as Mediators Between Gut Dysbiosis and Amyloid Pathology in Alzheimer’s Disease. J Alzheimers Dis. 2020;78(2):683-697.
5. Tache Y, Larauche M, Yuan PQ, Million M. Brain and Gut CRF Signaling: Biological Actions and Role in the Gastrointestinal Tract. Curr Mol Pharmacol. 2018;11(1):51-71.
6. Mayer EA, Tillisch K. The brain-gut axis in abdominal pain syndromes. Annu Rev Med. 2011;62:381-96.
7. Peters SL, Yao CK, Philpott H, Yelland GW, Muir JG, Gibson PR. Randomised clinical trial: the efficacy of gut-directed hypnotherapy is similar to that of the low FODMAP diet for the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2016 Sep;44(5):447-59.
8. Peter J, Fournier C, Keip B, Rittershaus N, Stephanou-Rieser N, Durdevic M, Dejaco C, Michalski M, Moser G. Intestinal Microbiome in Irritable Bowel Syndrome before and after Gut-Directed Hypnotherapy. Int J Mol Sci. 2018 Nov 16;19(11):3619.
9. Lacy BE, Pimentel M, Brenner DM, Chey WD, Keefer LA, Long MD, Moshiree B. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Am J Gastroenterol. 2021 Jan 1;116(1):17-44.
10. Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013 Nov 15;74(10):720-6.